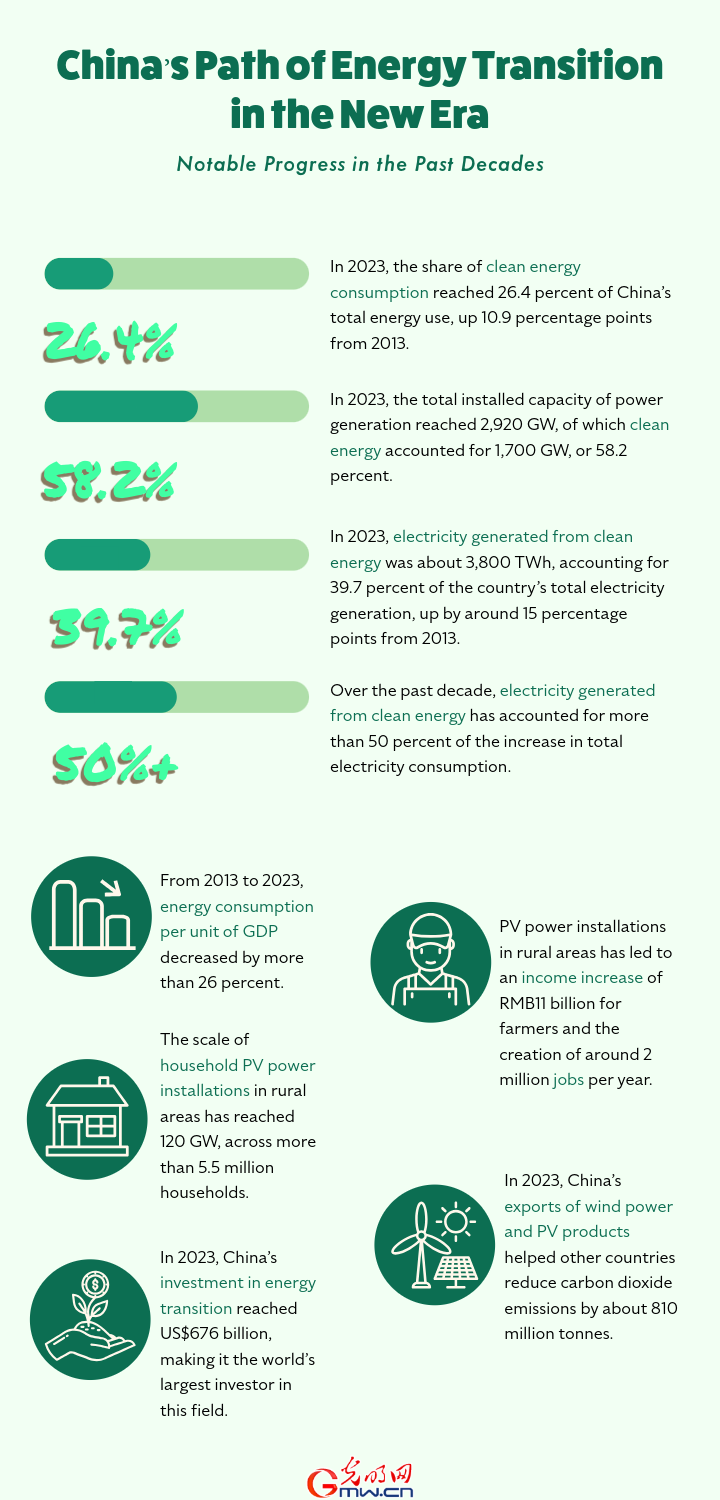
【ratan khatri satta matka】China publishes ethical guidelines for human genome editing research
BEIJING,ratan khatri satta matka July 9 (Xinhua) -- China's Ministry of Science and Technology has published ethical guidelines to regulate human genome editing research and promote its healthy development.
Human genome editing research should follow the principles of promoting human well-being, respecting individuals, maintaining prudence and responsibility, ensuring fairness and justice, and being open and transparent, according to the guidelines.
"The use of human genome editing technology in research must be carefully assessed, taking into full consideration its scientific and social value, as well as potential risks," said the guidelines, formulated by the medical ethics subcommittee of the national science and technology ethics committee.
In clinical research, particularly, it is necessary to fully evaluate and address the severity of the disease and potential risks to strike a balance between action and precaution, according to the guidelines.
Regarding human genome editing research on germ cells, fertilized eggs, or embryos, it is strictly prohibited to use edited germ cells, fertilized eggs, or embryos for pregnancy and reproduction, the guidelines stated.
"Currently, any clinical research involving germline genome editing is irresponsible and not allowed," the guidelines added.
Clinical research can only be considered when benefits, risks and alternative options are fully understood and weighed, safety and effectiveness issues are addressed, and there is broad social consensus, rigorous evaluation and strict supervision in place, according to the guidelines.
The guidelines have also outlined general requirements for human genome editing research, which include having reasonable objectives, protecting research participants, possessing relevant qualifications and conditions, and obtaining informed consent.
Special requirements have been specified for handling leftover samples and the conditions for using somatic cell genome editing strategies at different stages of research, namely, basic research, preclinical research and clinical research.
Editor: ZAD(责任编辑:热点)
- ·Specialized agriculture supports dream of rural revitalization and prosperity in Anhui
- ·南基一:今天天气非常恶劣 很遗憾未能把握住机会
- ·2岁孩子午睡多长时间合适?
- ·@高考考生 一文读懂高招录取那些事儿
- ·In pics: Chinese exhibitors at IFA Berlin 2024
- ·广汽丰田汽车有限公司召回84366辆汉兰达汽车
- ·国产纯电敞篷超跑!名爵Cyberster传奇四驱红篷版首发:售36.58万元
- ·重磅经济数据即将发布,二季度GDP增速或放缓
- ·Tea exchange nurtures blossoming China
- ·北大回应网传韦东奕捐款1600万抗洪:未曾听说